Abstract
Introduction: COVID-19 pandemic has posed multiple challenges for allogeneic haematopoietic cell transplantation (HCT) practices. In order to ensure graft availability before the start of chemotherapy/radiotherapy conditioning regimens, national and international bodies recommended HSC cryopreservation. This led to unprecedented numbers of allogeneic HCT with cryopreserved grafts and highlighted the need to establish the effects it may have on patient outcomes. Previously available evidence, although re-assuring, is not conclusive.
Methods: All adult and paediatric patients receiving allogeneic transplant (excluding cord blood) in UK transplant centres between 1st June 2020 and 31st August 2021 were eligible for the study. Fresh graft HCTs from the same period were controls. Patient demographics were compared by Fisher's exact test for binary comparisons; by the Wilcoxon rank sum test for continuous variables and by logistic regression for ordered categorical variables. Patient outcomes (OS, PFS, neutrophil and platelet recovery) were calculated by the Kaplan-Meier method. NRM and RR were calculated by competing risks, with the competing event for NRM being relapse and for RR death in remission. Cox multivariable comparisons between recovery times, OS, PFS and cGvHD were made by Cox regression. Univariate and multivariate comparisons of NRM and RR were by competing risks regression.
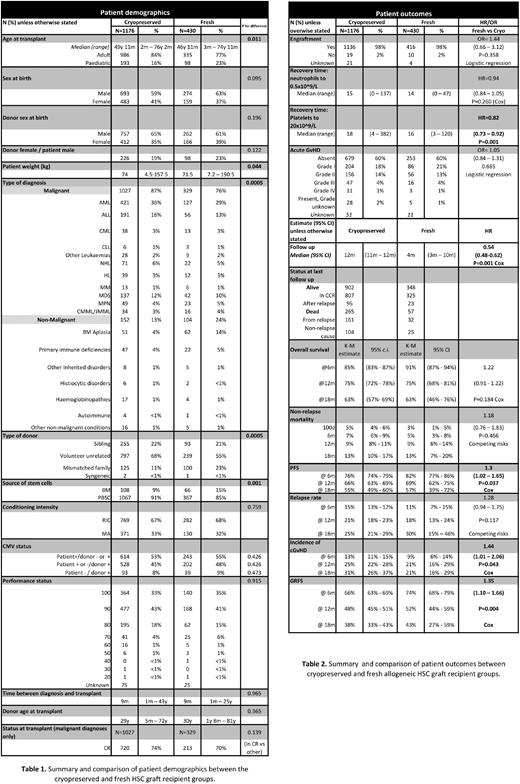
Results: Data were available for 1606 allogeneic HSC transplants performed at 29 centres accounting for 89% of all allogeneic HCT performed during the study period in the UK. 1176 patients received cryopreserved and 430 had fresh grafts. Patient characteristics are summarised and compared in Table 1. Of note, more BM harvest grafts were transplanted fresh than cryopreserved (15% vs 9%, p=0.01). Proportion of patients receiving reduced intensity conditioning (RIC) versus myeloablative (MA) were comparable between the groups (p=0.76).
Patients receiving cryopreserved HCT were older (median: 49 years 11 months vs 46 years 11 months, p=0.01), of higher weight (median 74 kg vs 71.5 kg, p=0.04) and were more likely to receive the HCT for malignant indication (87% vs 76%, p=0.01). There was a higher proportion of PBSC (91% vs 85%, p=0.01) and unrelated donor grafts (68% vs 55%, p=0.01) in the cryopreserved group. Median follow-up was longer for patients receiving cryopreserved grafts (12 months vs 4 months, p=0.01).
Patient outcomes are summarised in Table 2. Neutrophil engraftment was 15 days (range, 0-137) and 14 (range, 0-47) for patients receiving cryopreserved and fresh grafts, respectively (p=0.26). There was a statistically significant difference in platelet recovery favouring fresh grafts: 16 days (range, 3-120) in comparison to 18 days (range, 4-382) for cryopreserved grafts (p=0.01).
Cryopreservation did not have a detectable effect on OS, RR and NRM (p=0.18, p=0.47 and p=0.12, respectively). PFS was worse for patients receiving cryopreserved grafts (HR 1.3, 95% CI: 1.02-1.65, p=0.04). In a multivariate analysis, cryopreservation remained a significant contributing factor for shorter PFS (HR 1.34, 95% CI: 1.02-1.74, p=0.03) together with malignant indication for transplantation, RIC and lower Karnofky's score at the time of transplant.
Cumulative incidence of cGvHD was higher in patients receiving cryopreserved grafts (HR 1.44, 95% CI: 1.01-2.06, p=0.04) in the univariate but not in the multivariate analysis (HR 1.34, 95% CI: 0.91-1.98, p=0.14).
Conclusions: Our large multi-centre UK study suggests that allogeneic HSC graft cryopreservation delays platelet engraftment and may impact PFS. However, during this COVID-19 period, there were important differences in patient and transplant characteristics between those HCTs using fresh and cryopreserved grafts, which may have contributed to the effects seen. For that reason, comparison of patient outcomes using cryopreserved grafts (Jun 2020 - Aug 2021) and fresh grafts from a pre-pandemic cohort (Jun 2018 - Aug 2019) is also being compiled and will be presented. When deciding whether to use fresh or cryopreserved allogeneic HSC grafts, there must be a careful risk versus benefit assessment for each specific patient-donor pair. Robust back-up plans should be in place if fresh graft would be considered.
Disclosures
Snowden:Mallinckrodt: Speakers Bureau; Janssen and Jazz: Speakers Bureau; Gilead: Speakers Bureau; Novartis: Speakers Bureau; Medac: Membership on an entity's Board of Directors or advisory committees; Kiadis: Other: clinical trial IDMC membership .
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal